- Prevention
Vascular co-option
- Is there any realistic opportunity to develop preventive strategies against metastasis?
Vascular co-option
García-Gómez P and Valiente M. (2020). Tumor vascularization. Elsevier.
Vascular co-option in brain metastasis.
García-Gómez P and Valiente M. Angiogenesis. (2020).
Serpins promote cancer cell survival and vascular co-option in brain metastasis.
Valiente M et al. Cell. (2014).
Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization.
Er E et al. Nature Cell Biology. (2018).
A clinically-compatible drug-screening platform based on organotypic cultures identifies vulnerabilities to prevent and treat brain metastasis.
Zhu L, et al. EMBO Molecular Medicine. (2022).
Protocol to generate murine organotypic brain cultures for drug screening and evaluation of anti-metastatic efficacy
Lucía Zhu, Lauritz Miarka, Patricia Baena, María Perea-García, Manuel Valiente. STAR Protocols (2023)
We realized that metastasis initiation in the brain and elsewhere require the interaction with pre-existing vessels. This physical interaction is termed vascular co-option. If we get to know the molecular machinery, we may get the opportunity to kill metastasis initiating cells in potentially every organ, however there are difficulties to apply a preventive strategy: how do we know when to start treating? When to stop treating? Maybe when the primary tumor is diagnosed is already too late since metastatic cells might have already disseminated and formed tumors in other organs, even if they are still small and asymptomatic. So, a critical point to prevent metastases is really to design a realistic window of opportunity where we can perform experimental approaches that are clinically relevant.
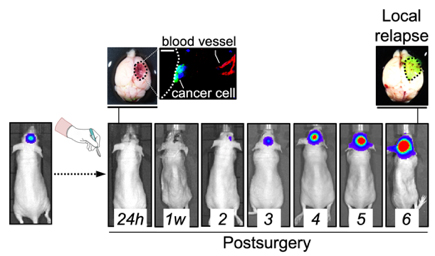
We have modelled clinically relevant scenarios that involve tumor/ metastasis reinitiation such as, for instance, relapse post-therapy. After neurosurgery, the established brain metastasis is dramatically reduced but there are always cancer cells left behind that can survive and might eventually re-grow the tumor. We hypothesize that regrowing the tumor would involve mechanisms required during naïve metastasis initiation, including vascular co-option, in additional to other relapse-specific aspects that will be relevant only in this context (i.e. tissue damage, inflammation). Thus, we hope the model of relapse post-surgery we have established will allow us to characterize these cancer cells left behind to then act pharmacologically on them to prevent relapse. If successful, we can test this approach in spontaneous models of metastasis to understand when we should start the treatment respect to the presence of the primary tumor and for how long we should maintain it. These experimental approaches will be critical to build a solid and clinically-compatible preventive strategy for metastasis.
We envision that exploiting vascular co-option is a valuable strategy to prevent metastasis.