- The brain with metastasis
Neurons
- Is it possible to limit the impact of metastasis on brain function?
Brain metastasis models: what do we need to aim for better treatments.
Masmudi-Martín M, Zhu L et al. Advanced Drug Delivery Reviews. (2021).
Viewpoint: Brain Metastasis
Boire A, Brastianos P, Garzia L and Valiente M. Nat Rev Cancer. (2020).
Protocol to generate murine organotypic brain cultures for drug screening and evaluation of anti-metastatic efficacy
Lucía Zhu, Lauritz Miarka, Patricia Baena, María Perea-García, Manuel Valiente. STAR Protocols (2023)
Machine learning identifies experimental brain metastasis subtypes based on their influence on neural circuits.
Sanchez-Aguilera A**, Masmudi-Martín M**, Navas-Olive A, Baena P, Hernández-Oliver C, Priego N, Cordón-Barris L, Alvaro-Espinosa L, García S, Martínez S, Lafarga M, RENACER, Lin MZ, Al-Shahrour F, Menendez de la Prida L* and Valiente M*. Cancer Cells.
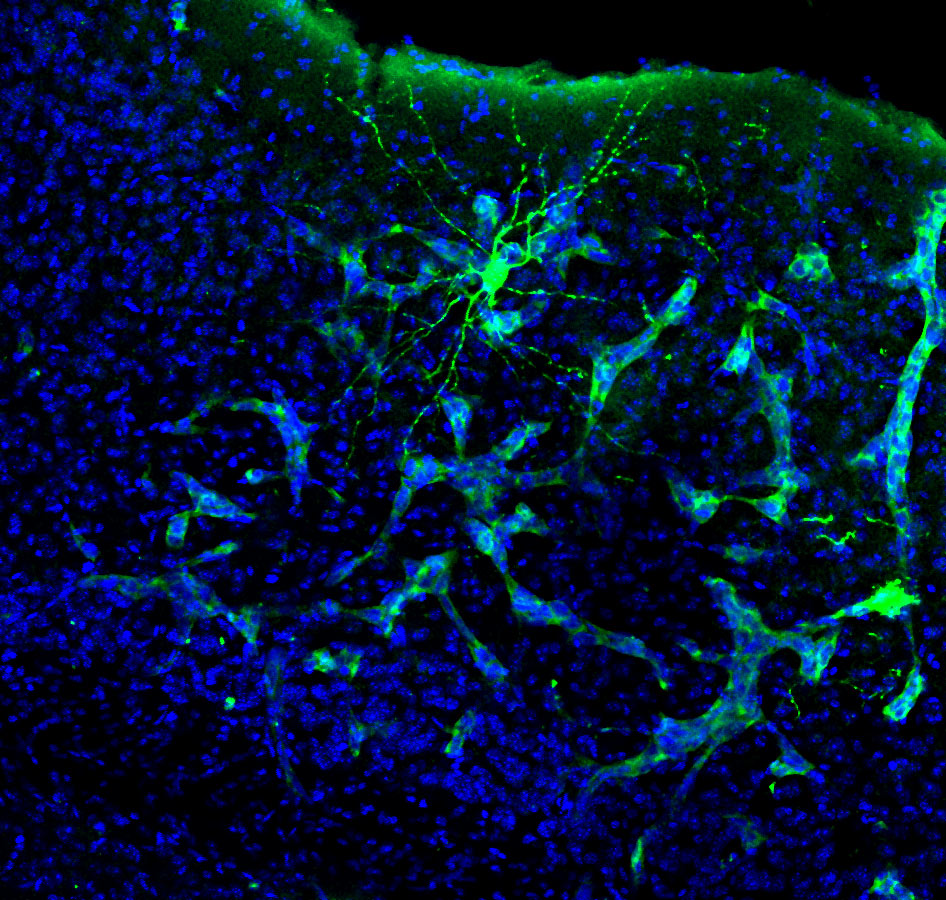
Almost half of the patients with brain metastasis have to face a variable degree of compromise on their neurocognitive functions. Obviously, this aspect might have a terrible impact in their quality of life. Surprisingly, our knowledge on the molecular aspects behind this negative influence of metastasis on neural circuits is literally absent. Indeed, attending to the limited clinical studies performed on this subject, it seems that the mass effect cannot explain by itself why some patients have a neurocognitive compromise while others do not.
We have partnered with neuroscientists to apply technologies that can measure neural circuit activity on our brain metastasis models, which we aim to assign to specific degrees of neurocognitive impairment. Besides studying brain electrophysiology in awake mice affected with metastasis performing specific tasks, measuring the changes in cellular excitability following calcium reporters in neurons and glial cells as well as the potential influence in specific behavioral tests as a surrogate of neurocognitive evaluation, we also apply Raman spectroscopy with advanced next-generation low invasive probes (see Nanobright) as a novel approach to learn about potential altered patterns that could, in the future, be used to identify the emergence of this negative impact of brain metastasis. All these experimental approaches are being developed in parallel with an ambitious program on human brain metastasis aiming to explore the molecular similarities among those metastases that had been clinically annotated to cause neurocognitive disfunction in the patient versus others that did not develop such clinical symptoms.
Our goal is to dissect the molecular crosstalk between metastasis and neuronal circuits, which might involve neurons and glial cells, and to correlate it with the origin of neurocognitive disfunction. By doing so we expect to find a new set of therapeutic strategies to alleviate and even prevent the compromise of brain metastases on the quality of life of patient by protecting brain function from the influence of the tumor.