- The brain with metastasis
Astrocytes
- What is the nature of brain metastasis-associated astrocytes?
Serpins promote cancer cell survival and vascular co-option in brain metastasis.
Valiente M et al. Cell. (2014).
STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis.
Priego N et al. Nature Medicine. (2018).
The potential of astrocytes as immune modulators in brain tumors
Priego N and Valiente M. Frontiers in Immunology. (2019).
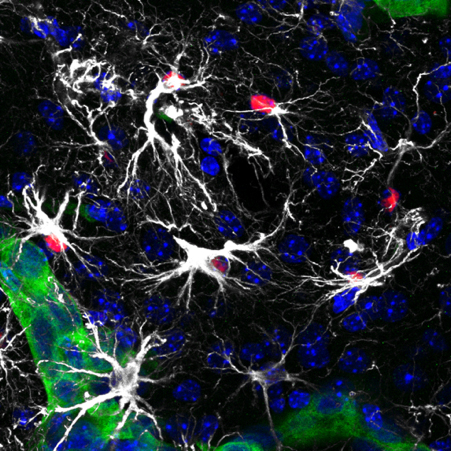
The most abundant brain cell type becomes reactive when sensing a thread that they try to eliminate to keep brain function unaltered. However, a few metastatic cells escape this natural defence and rewire the glial cell anti-tumor function into a pro-metastatic niche. Thus, becoming a target for brain metastasis.
To understand the biology of brain metastasis-associated reactive astrocytes we use a broad variety of techniques including single-cell omics, conditional GEMM, cell type specific adeno-associated viruses, calcium imaging, 3D astrospheres, primary cultures, in vivo electroporation to generate a full characterization of this critical component of the microenvironment.
We have learnt about the importance of deconstructing glial cell heterogeneity by identifying pSTAT3+ reactive astrocytes as a critical contributor to brain metastasis. These astrocytes co-exist with other that are pSTAT3- reactive astrocytes in the peritumoral margin of metastases, independently of their origin. This fascinating discovery has been translated into a novel therapeutic application validated in patients with brain metastasis, emphasizing the importance of targeting the metastasis through the microenvironment.
We are deconstructing this signalling pathway within microglia/macrophages not only to understand the underlying biology but to explore whether we can target it. We hypothesize that impairing the microenvironment crosstalk is critical to impair the formation of a pro-metastatic environment in the brain.